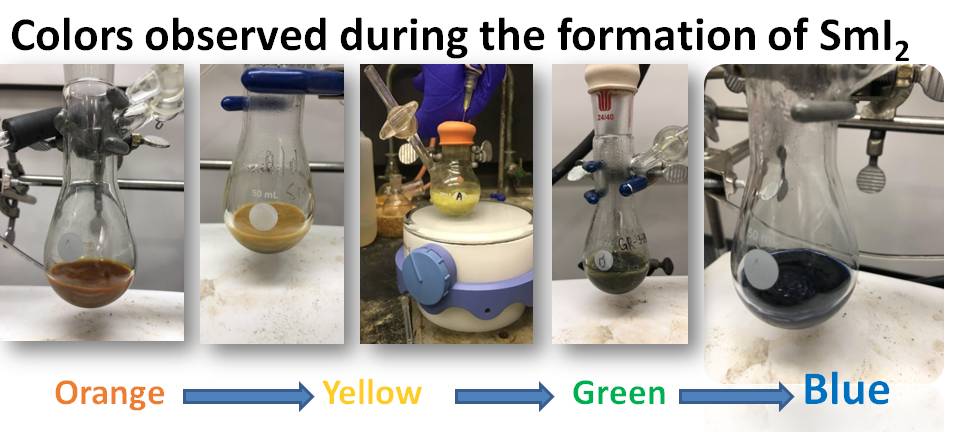
Samarium diiodide (SmI2) is a versatile single-electron reductant which can reduce various functional groups under mild reaction conditions and facilitate radical and nucleophilic carbon-carbon bond-forming events. It was realized as a synthetic tool in the early 1980’s, and since then various studies have been carried out to understand the types of functional groups SmI2 can reduce, as well as the rates at which these reductions take place.
SmI2 behaves as a reductant in its +2 oxidation state, and when it is exposed to air it is rapidly oxidized to its non-reactive +3 oxidation state. This air-sensitivity is easily managed by carrying out SmI2 reactions under inert Argon atmosphere. In industry and many large universities gloveboxes can be used with SmI2 as a reagent with reasonable ease; however, many labs do not have this equipment. While it ranks as one of the top synthetic tools to organic chemists, if research chemists are intimidated by its need for specialized equipment the reagent will become underutilized. For this reason, one of the goals of many of the top SmI2 researchers is to make it clear to the researching public the ease of SmI2 use in the lab. This becomes an even larger hurdle when working in primary undergraduate institutions, where inert atmosphere equipment, like a glovebox, may not be standard.
A lower cost alternative to a glovebox is access to a vacuum pump, argon tank and Schlenck line glassware. The goal in our lab is to work through the details of the synthesis, identify the most important factors and write a protocol for the formation and use of SmI2 using a Schlenck line oxygen-free technique. The only way that SmI2 can become a more common reagent with synthetic chemists is to communicate how everyone can use this reagent properly even without more sophisticated equipment.
PROJECT 2. SmI2/Ni(II): in situ generation of Ni(0) and application in the NHK reaction.
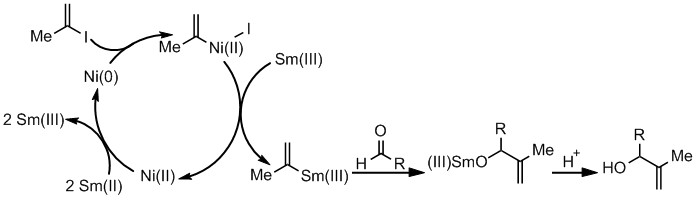
SmI2 in THF can carry out the reduction of various functional groups through single-electron reduction; however, the unique selective reactivity that leads to interesting bond-forming events in coupling reactions has been observed with the inclusion of additives. A common additive used to enhance sluggish SmI2 reactions is catalytic Ni(II). Since 2010, a clear mechanistic picture of how Ni(II) works with SmI2 was identified.
Many cross-coupling reactions traditionally carried out with Pd(0) catalysts are being redeveloped using Ni(0). This change in catalyst benefits from significantly lower costs of the readily available metal, and increased reactivity of Ni(0) with different leaving groups. Reactions which proceed through a Ni(0) intermediate are still limited by the means in which Ni(0) is introduced into the system. With the understanding that SmI2 with catalytic Ni(II) provides a mild, facile means of forming Ni(0) in situ, more reactions which require Ni(0) as the active catalyst can be explored. This project focuses on adapting the Sm/Ni(II) system to new reactions which will expand the scope of carbon-carbon bond forming methods.